Batteries for dummies part 2
Those of you who read my blog post „Batteries for dummies part 1“ might look forward to the second part. Let’s start with a short repetition:
In the last post we learned about batteries is and that they consist of interconnected secondary cells that are rechargeable. We also learned that the total energy content consists of the product of voltage and capacity. After that, we calculated the energy content of a battery brick and got to know all sorts of batteries. But let’s get down to today’s input: it’s all about chemistry.
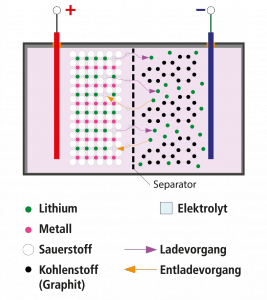
What happens when we charge a battery? In a nutshell: Electrical energy is transformed into chemical energy. When you connect an electrical consumer (like a LED or a motor) to the battery, chemical energy will be converted into electrical energy again.
When charging a battery, current flows through it and this leads to a chemical reaction inside the battery where positive and negative electrodes chemically change. Once the battery is fully charged we can extract the added current. Again, this will lead to a chemical reaction, however, in reverse order. Current can be extracted from the battery as long as there is a chemical reaction possible.
When being at trade shows, like the Intersolar in Munich, the terms nominal voltage, efficiency voltage and energy density are frequently used. But what exactly do they mean?
The nominal voltage of an electrical consumer or a voltage source (the power grid, for example) is the voltage’s value in normal operation. The European nominal voltage is 230V. The nominal voltage of a lithium ion battery is between 3.2 and 3.8V per cell.
We will deal in more detail with the term “efficiency voltage” in one of the next blog posts: the term describes the relation between the amount of energy that is emitted during the battery’s discharging process (called useful energy) and the amount of energy that is added during the charging process. Lithium ion batteries have one of the best efficiency voltages: about 90%. This means, there is a power loss of only 10%.efficienta
Energy density is the amount of energy with regard to height. For example, you can see how much energy can be accumulated in a cubic meter of space. A lithium ion battery has an energy density of 120-210Wh/kg (watt-hours per kilogram). Compared to a lead accumulator that has an energy density of about 30 watt-hours/kg, this is relatively high.
Now we have a closer look at the features, which the battery brick provides. There is one interesting advantage: by using the “jumper” (the little black connector), we can set the battery brick at 9V or 12V. When the battery brick is connected to the solar module it should always be set to 12V. Usually, the brick system is designed for 9V, however, the bricks included in the set withstand 12V.
There are two brick voltage regulators (ALL-BRICK-0300 and ALL-BRICK-0299) at Brick’R’knowledge that make it possible to get exactly 9V out of the solar module. But let’s return to the battery brick: It has a input voltage of 8-15V and an input current of max. 400mA. The output voltage can be set to 8.5V or 11.5V because, as we have learned, this battery does not have a 100% efficiency. At 11.5V output voltage the output current is at 500mA, at 8.5V it is 750mA. You might have noticed that the LED blinking varies when connecting the battery. The LED’s different colors tell you what the battery is doing. Here you can read about the meaning of the different colors.
Finally, we have to calculate again. We haven’t considered one thing: watt= voltage x current. When you unload the battery with a current flow of 111mA and a nominal voltage of 9V, this corresponds to an electrical power conversion of 1 watt-hours, meaning: 0,111A x 9V = 1W.
As the battery’s performance is 16Wh and the electrical power conversion is 1W, the brick battery could operate the Highpower LED brick for 16 hours until it is empty.
For those of you who find this blog post interesting: there will soon be a series of blog posts regarding photovoltaic systems.
Stay tuned!

 en
en de
de es
es